Zircon
Zircon
Zirconium is a chemical element (symbol Zr). Zirconium is a non-toxic heavy metal in the titanium group of the periodic table.
Zircon (also referred to as zirconium silicate), ZrSiO4, is a naturally occurring mineral found in many colours, which is used a gem; the colourless version is sometimes used as a diamond substitute. Chemically it is a mixture of varying ratios of silicon dioxide (SiO2) and zirconium dioxide (ZrO2), generally with hafnium. Zircon sand is used as the raw material for manufacturing zirconium dioxide.
Zirconia (ZrO2) is a transparent diamond imitation gem, manufactured synthetically from zirconium dioxide. More precisely it is CSZ, cubic stabilised zirconium dioxide at very high temperatures (above 2370°C) due to the addition of other metal oxides. When stabilisation involves using yttrium oxide the term is YSZ and with calcium oxide it is CaSZ.
Zirconium dioxide (ZrO2), also zirconium(IV) oxide, zircon oxide, (previously also referred to as zircon acid or zirkonerde [zirconia]) is a high performance oxide-ceramic. The naturally occurring mineral in monoclinic (at room temperature to 1173°C crystallising) form (modification) is called baddeleyite.
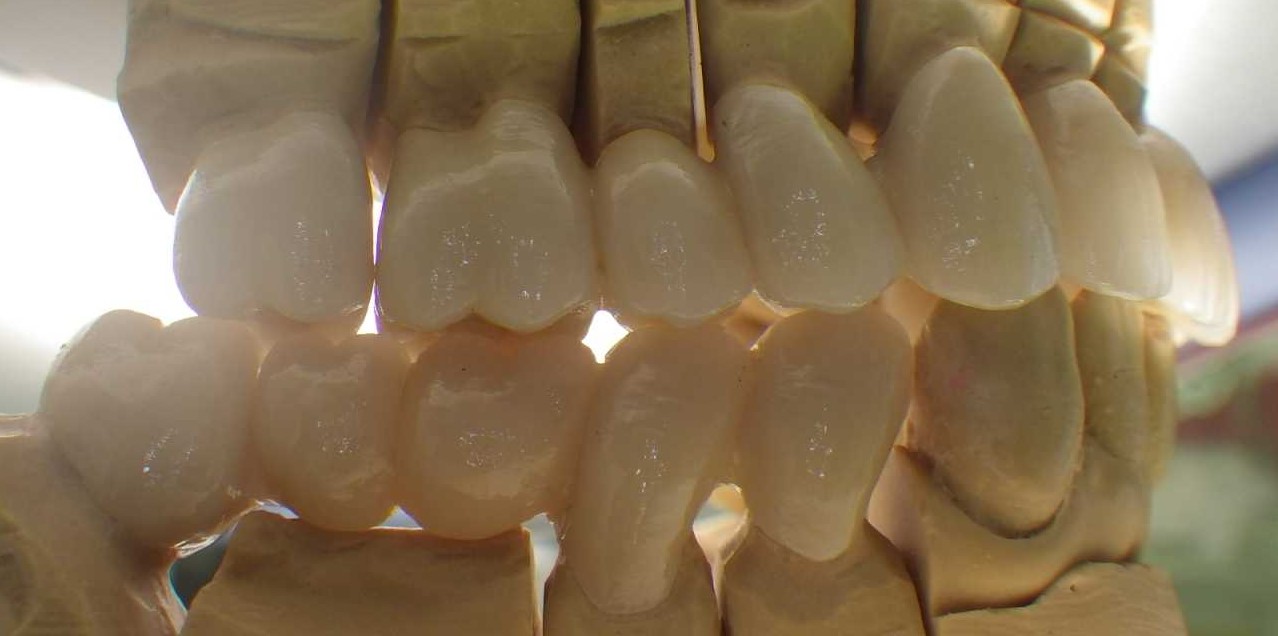 All-zirconia bridgework and crowns
All-zirconia bridgework and crowns
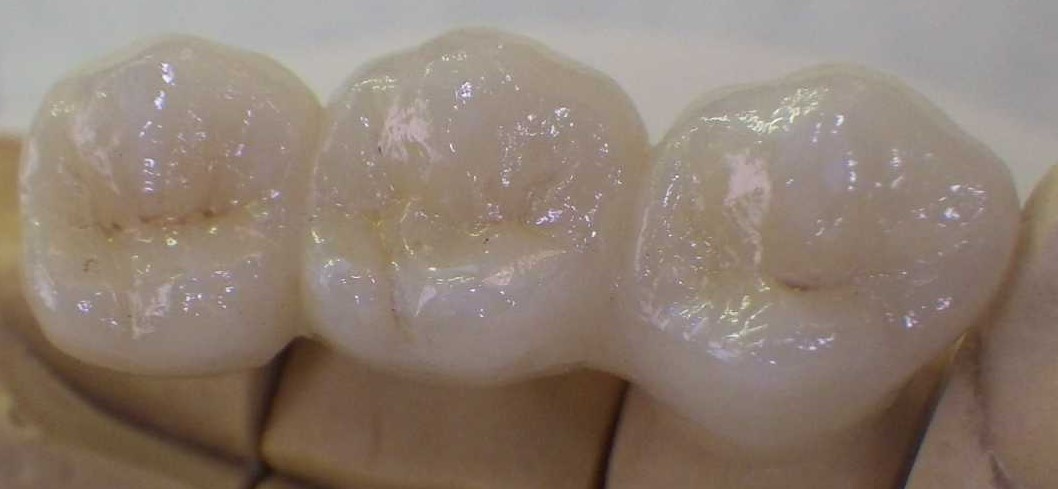 Zirconium dioxide free-end bridge
Zirconium dioxide free-end bridge
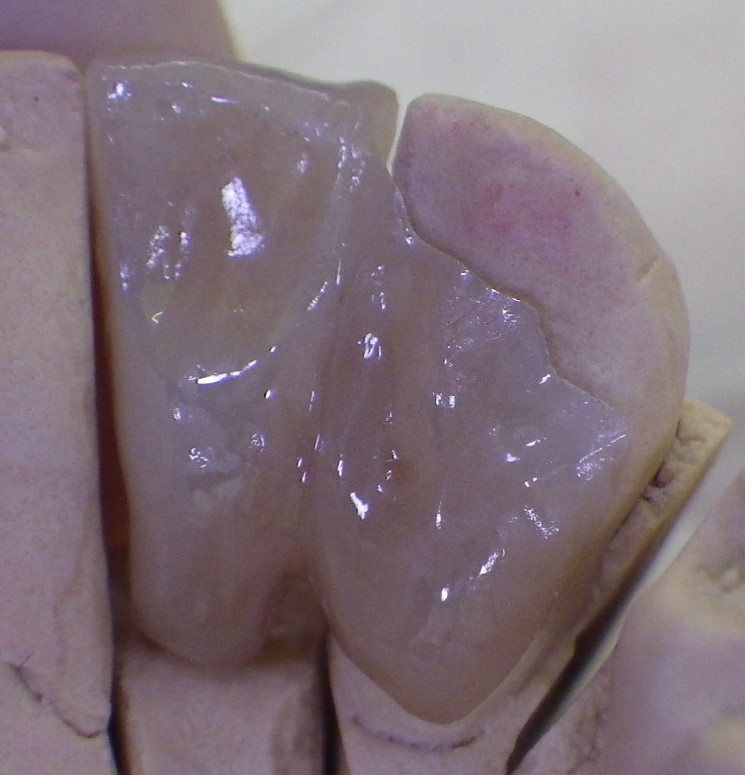 Lingual view of single-winged bridge
Lingual view of single-winged bridge
Compaction of highly pure zirconium dioxide powder and binding agents without heat treatment produces a press blank, known as a green compact or green body. Shrinkage of 25% at a later stage must be taken into account. A presintering process is then completed at approx. 1000°C, during which the binding agents are eliminated, shrinkage of approx. 5% occurs. This produces a partially sintered blank. In this partially sintered state the zirconium dioxide can be processed using many conventional dental CAD-CAM milling systems. Subsequent shrinkage of 20% at the (post) sinter firing stage must be factored into the calculation by respective enlarged manufacturing.
Alternatively, the zirconium dioxide can also first be densely sintered and then hot isostatically pressed (recompacted). The resultant material undergoes no further shrinkage and can therefore be processed dimensionally accurately (1:1), however, this material is extremely hard and the tool wear exceptionally high.
CeSZ zirconium dioxide partially or fully stabilised with cerium oxide.
CaSZ zirconium dioxide stabilised with calcium, form of zirconia.
CSZ cubic stabilised zirconium dioxide (above 2370°C) TSZ tetragonal stabilised zirconium dioxide (temperature range 1173°C to 2370°C)
YSZ zirconium dioxide partially or fully stabilised with yttrium oxide.
PROBIEREN SIE ES EINFACH AUS !!!
Von uns erhalten Sie professionelle Unterstützung.
Treten Sie mit uns in Kontakt oder nutzen Sie unser Kontaktformular.
Wort des Tages
Schwerpunkttext des Monats
Vergrößernde Optik in der Zahntechnik Vergrößernde Optik in der Zahntechnik |
